17+ Calculate The Molar Heat Of Solution For Magnesium Sulfate
The temperature of the water rises from 2135c to 266c. Mass of 50 mL beaker Nacl 336571g Thus the molar heat capacity of any substance is defined as.
19 Enthalpy Of Solution For Mgso4 S And Mgso47h2o S Are 20 3kcal And 4 8kcal Calculate Hydration Enthalpy Of Mgso4
Calculate the heat of solution AH goln Specific heat of the solution SH0961 calgC and molar mass of MgSO4 MW120366 gmol.

. The units of molar heat capacity are. 2 Moles is multiplied by the molar heat of solution. The heat of solution of the oxysulfate compound in.
Simply grind the MgSO4 7H2O Magnesium Sulfate Heptahydrate Epsom Salts into a fine powder first then spread onto large baking sheets for maximum surface area. Joules per degree Celsius per mole J C-1 mol-1. The solubility of the oxysulfate is given in the form of a plot of the basicity and MgSO 4 content of magnesium sulfate solutions.
So Molar mass of MgSO4 Molar mass of 1 Magnesium Mg atom Molar mass of 1 Sulfur S atom Molar mass of 4 Oxygen O atoms. 24305 3206 15999. Give your answer for q values as it.
Combustion of 10 g magnesium produces 16 g magnesium oxide. Homework Statement When 500 g of NaOHs are added to 100 g of water using a calorimeter with Cp 49324 JK the temperature rises from 250 to 375 C. To determine the molar mass of a compound we multiply the molar mass of each element by the.
Sulfuric acid magnesium salt 11. 5g of Magnesium sulfate dissolves in 100g of water in a coffee-cup calorimeterconstant pressure. The information given lacks but nevertheless the answer is.
Magnesium sulfate Permanent link for this species. 3 The joules of heat released in the dissolution process is used with the specific heat equation and the total mass. Calculate heat change of metal.
Equivalent molar concentration per liter. To understand Enthalpies of Solution and be able to use them to calculate the Heat absorbed or emitted when making solutions. Density of magnesium sulphate is equal to 2.
1 mass of magnesium sulfate 42 g Molar mass of magnesium sulfate 120 gmol Number of moles of magnesium sulfate mass molar mass 42 g. Calculate amount of oxygen consumed when 020 g of magnesium was burned in air. So the total heat free by dissolving the solute was 1386 32 1417 J Then.
The molar mass of magnesium sulfate is 1203676 gmol. Use this link for bookmarking this species for future reference. The solution for this problem is.
Magnesium sulfate weighs 266 gram per cubic centimeter or 2 660 kilogram per cubic meter ie. Magnesium sulphate weighs 266 gram per cubic centimeter or 2 660 kilogram per cubic meter ie. Density of magnesium sulfate is equal to 2 660 kgm³.

Pearson Chemistry Y11 Pdf Chemical Reactions Periodic Table

Carbon Carbon Double Bond Shift In The Biosynthesis Of The Antibiotic Corallopyronin A Corj Dh A Shift Domain

Flow Assurance Solids In Oil And Gas Production Pdf Mole Unit Petroleum Reservoir

Application Of Hess S Law To Determine The Enthalpy Change Of Hydration Of Magnesium Sulphate A Level Science Marked By Teachers Com

Enthalpy Change Of Solution Of Hydrated Magnesium Sulphate

Do Now Turn In Molar Quantities Lab Pick Up Notes Ppt Download
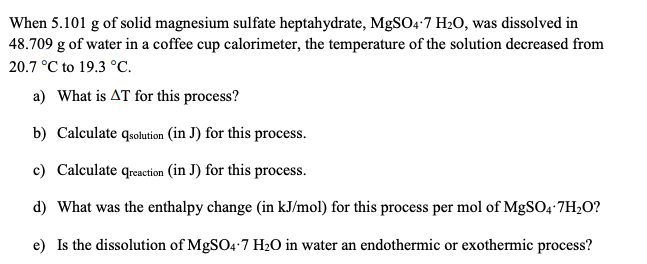
Solved When 5 101 G Of Solid Magnesium Sulfate Heptahydrate Chegg Com

If 0 7 Moles Of Barium Chloride Is Treated With 0 4 Mole Of Potassium Sulphate Number Of Moles Of Barium Sulphate Formed Is

Chemistry For The Ib Diploma Second Edition By Cambridge University Press Education Issuu
Solved A Magnesium Sulfate Mgso4 Is Added To 260 Ml Of 2 40 10 2 Course Hero

The Number Of Moles Of Magnesium Present In A Magnesium Ribbon Weighing 12 G Is Molar Atomic Mass Of Magnesium Is 24 G Mol

Solved 1 An Aqueous Solution Of Magnesium Sulfate Is Chegg Com
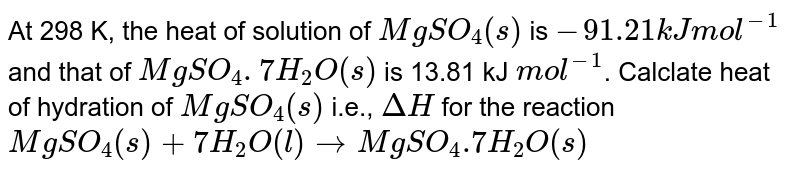
At 298 K The Heat Of Solution Of Mgso 4 S Is 91 21 Kj Mol 1 And That Of Mgso 4 7h 2 O S Is 13 81 Kj Mol 1 Calclate Heat Of Hydration Of Mgso 4 S I E Deltah For

Schaum S Outlines 3 000 Solved Problems In Chemistry Pdf Chemical Bond Gases

Solved 3 When 5 101 G Of Solid Magnesium Sulfate Chegg Com

Enthalpy Change Of Solution Of Hydrated Magnesium Sulphate

Solved 3 4 01 G Of Mgso4 Is Placed Into 80 0 Ml Of Water The Waters Temperature Increases By 6 45 C Calculate Hin Kk Mol For The Dissolution Of Mgso4 The Specific Heat